What Is Acid Rain?
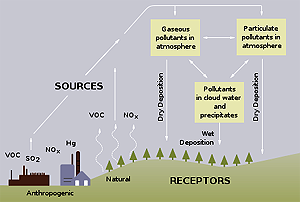
The term "acid rain" is commonly used to mean the deposition of acidic components in rain, snow, fog, dew, or dry particles. The more accurate term is "acid precipitation." Distilled water, which contains no carbon dioxide, has a neutral pH of 7. Liquids with a pH less than 7 are acid, and those with a pH greater than 7 are alkaline (or basic). "Clean" or unpolluted rain has a slightly acidic pH of 5.6, because carbon dioxide and water in the air react together to form carbonic acid, a weak acid. Around Washington, D.C., however, the average rain pH is between 4.2 and 4.4.
The extra acidity in rain comes from the reaction of air pollutants, primarily sulphur oxides and nitrogen oxides, with water in the air to form strong acids (like sulphuric and nitric acid). The main sources of these pollutants are vehicles and industrial and power-generating plants. In Washington, the main local sources are cars, trucks, and buses.
The primary causes of acid rain are sulphur dioxide and nitrogen oxides. These chemicals are released by certain industrial processes, and as a result, the more industrialized nations of Europe as well as the US suffer severely from acid rain.
Acid rain causes acidification of lakes and streams and contributes to the damage of trees at high elevations (for example, red spruce trees above 2,000 feet) and many sensitive forest soils. In addition, acid rain accelerates the decay of building materials and paints, including irreplaceable buildings, statues, and sculptures that are part of our nation's cultural heritage. Prior to falling to the earth, sulphur dioxide (SO2) and nitrogen oxide (NOx) gases and their particulate matter derivatives — sulphates and nitrates — contribute to visibility degradation and harm public health.
|